Collagen peptides made from "the power of ginger"
Ginger, used for its unique spiciness and aroma, is also known for containing the proteolytic enzyme,
zingibain. Nippi has found that this enzyme has the property of acting on hydroxyproline, which is a
characteristic amino acid in collagen proteins. Utilizing the power of this enzyme contained in ginger, we
established a special product specification, along with an efficient production method for collagen peptides
products containing a large amount of X-Hyp-Gly-type tripeptides.
Degradation of collagen by ginger enzyme

Ginger Hydrolyzed Collagen Peptides Product A Product B Product C Product D Product E
Stability of X-Hyp-Gly type tripeptides in blood
X-Hyp-Gly-type tripeptides are stable in blood. In addition to high absorbency in humans and mice, our in-house tests
have confirmed that X-Hyp-Gly-type tripeptides are transported to each tissue in the body without decomposing. For
this reason, X-Hyp-Gly-type tripeptides are considered more effective than other peptides.
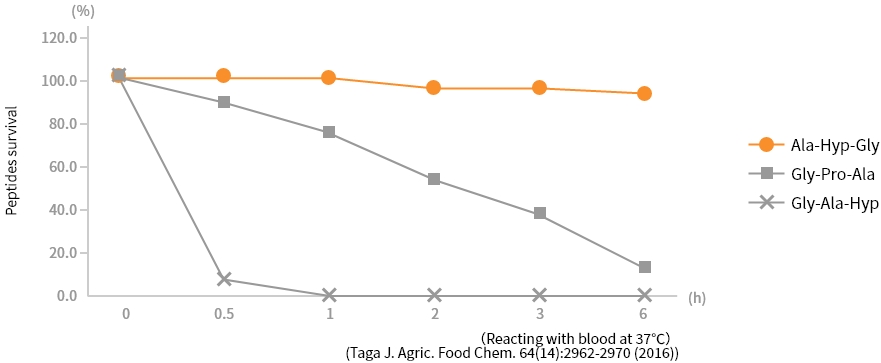
Blood stability of Ala-Hyp-Gly
Program authorization
INFORMED INGREDIENT

Ginger Hydrolyzed collagen peptides “Nippi Peptide Collagenomics” is certified to meet the requirements and
standards of Informed Ingredients, Anti-Doping program provided by LGC.
Main physiological functions of X-Hyp-Gly-type tripeptides contained in ginger hydrolyzed collagen peptides
Ginger hydrolyzed collagen peptides have a standardized high content of X-Hyp-Gly-type tripeptides.
It has been established that X-Hyp-Gly-type tripeptides.
- Promote osteoblast differentiation
- Inhibit angiotensin-converting enzymes
More Info
Ginger hydrolyzed collagen peptides were discovered as a result of Nippi’s persistent R&D

At the Nippi Research Institute of Biomatrix (NRIB), we are focusing on product development, and also on fundamental
collagen research. Post-translational modification of collagen is one of our most
important subjects. In our studies of amino acids, modified collagen is often decomposed to some extent before
analysis. For the research, the enzyme protease that decomposes collagen according to a fixed rule is an important
tool. While researching proteases at NRIB, Nippi focused on two proteases that act on collagen. One is gingipain, a
collagenase produced by periodontal disease bacteria, which does harm by breaking down collagen in the gums. The
other is zingibain, which is a major collagenase contained in ginger. Although these two enzymes are similar in that
they break down collagen, their properties and origins are completely different. While investigating the structure
and properties of the degraded peptides after these two enzymes act on collagen, we found that ginger-originated
zingibain produces peptides with unique characteristics. This is when we started to develop a new peptide product by
applying zingibain.