About Nippi Research Institute of Biomatrix, NRIB
The Nippi Biomatrix Research Institute, NRIB, is promoting research into the extracellular matrix found in multicellular animals, and exploring biochemistry, in particular, cell biology and bioengineering research on the extracellular matrix centered on collagen. In recent years, we have also been tackling research and development in a wide range of fields that apply protein engineering.
Cells are the basic units of life, and the human body is made up of a vast number of cells. According to recent research*1, total cell count is 36 trillion cells in the male (weight: 70 kg), 28 trillion in the female (weight: 60 kg), and 17 trillion in the child (10 years old). It is estimated that approximately 20-30% of all human proteins comprise collagen.
The surfaces of cells are surrounded by an insoluble complex, extracellular matrix (ECM), which links together to form a structurally stable composite, contributing to the mechanical properties of tissues. The ECM functions as a reservoir for growth factors and bioactive molecules. The ECM is a dynamic structure that is actively metabolized, and it plays a crucial role in determining the basic behaviors and characteristics of cells, such as proliferation, migration, and differentiation, and in controlling cell functions. The ECM also regulates protein synthesis and degradation, allowing the body's tissues and organs to maintain homeostasis and function normally.
Collagen is a family of proteins with quite unique properties. The primary structure of collagen consists of a repeated -Gly-Xaa-Yaa- sequence. Glycine (Gly), the amino acid with the smallest side chain, appears every three residues; proline (Pro) appears frequently at the Xaa and Yaa positions. Collagen has a secondary structure that differs from typical alpha helices and beta- structures. A single polypeptide chain forms a left-handed polyproline-II like structure, and three of these chains come together to form a loose right-handed helix structure.
This is the basic triple helical structure of collagenous protein.
In the case of the typical fibrillar collagen, type I, this structure continues over a length of approximately 300 nm (0.3 µm), with 1,014 residues of the -Gly-Xaa-Yaa- sequence. The three polypeptide chains are assembled with one residue shifted in the molecular axis direction, and the glycine residues are located close to the center axis of the molecule. The side chains at the Xaa and Yaa positions are all exposed on the surface of the molecule.
In the case of type I collagen, the pro-α1(I) and pro-α2(I) chains, which are derived from the COL1A1 and COL1A2 genes, respectively, are synthesized in the endoplasmic reticulum as polypeptide chains are synthesized in the endoplasmic reticulum. The two pro-α1(I) chains and one pro-α2(I) chain associate at their carboxy-termini to form a triple-helical structure from the C-terminus, undergoing hundreds of post-translational modifications along the way.
Collagen has unique post-translational modifications that are rarely found in other proteins, such as hydroxyproline (Hyp) and hydroxylysine (Hyl). In addition, Hyl can be further modified by enzymes to add galactose or glucose.
Collagen molecules form collagen fibrils through interactions between the side chains on the surface of the collagen molecule. These fibrils then form bundles, which form the framework structure of connective tissue, such as bones, teeth, skin, tendons, ligaments, eyes, and arteries. Depending on the type of collagen, some form meshwork rather than fibrils, while others are associated with cell membranes. Cells and ECM send signals to each other so that the cells contained in the tissue can function normally.
When the control system of the collagen family of proteins for protein synthesis and proteolysis is disrupted, it may cause some diseases including rare genetic disorders in bone, cartilage or skin, osteoporosis, fibrosis and cancers.
The human body is deeply connected to collagen, extracellular matrix (ECM) and connective tissue at various stages, from development to growth and ageing. Wound healing, including blood coagulation, is also related to collagen. In connective tissue, which is mainly composed of collagen, many types of the immune system fight against pathogens.
The researchers at NRIB are striving to understand the relationship of these cells and ECM, and to discover the cause of diseases.
Aiming towards further development of medical and healthcare fields, NRIB engages in cooperative research in Japan, Europe, USA and Asia, and participates in academic meetings, both domestically and internationally.
*1: https://www.pnas.org/doi/10.1073/pnas.2303077120

Confocal Laser Scanning Microscope
|

CD spectrometer
|
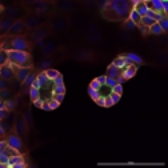
Cysts of MDCK cells in 3D gel
|
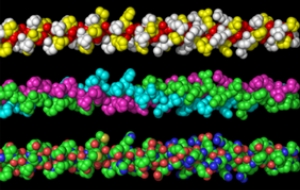
collagen structure
|

fluorescence microscope
|